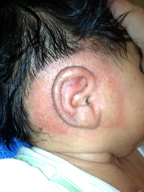
Skin redness is a common byproduct of the conduct of an EarWell™ procedure. The combination of the Cradle’s adhesive, Alcohol Wipes and Acetone/Alcohol Pads (or Swabsticks) are frequently abrasive to the infant skin. Mild redness following removal of the device and after skin prep is common and not of concern.
The adhesive used to attach the EarWell device is proprietary to Becon Medical Ltd., and was developed together with the 3M™ Company. It is a porous, hypoallergenic, continuous contact tape that has been extensively human tested for biocompatibility and biotoxicity.
There have been few reports of allergies despite its use on thousands of infants. When the rare allergy is observed, it typically is evidenced by a premature release of the adhesive with an underlying moist and erythematous skin. The skin may also have a rough and scaly texture, similar to that of eczema. The majority of these occurrences have been among infants with more severe “infant acne” or seborrhea (cradle cap), i.e., infants who already have more sensitive skin types. These reactions will not typically become evident until after one or two cradle applications.
 |
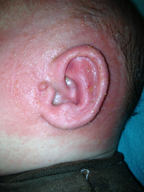 |
EarWell physicians recommend the tid application of a thin layer of over-the-counter hydrocortisone cream. The redness will generally clear in 48-72 hours. EarWell treatment should be terminated. The parents are encouraged to keep the ears secured with an infant headband when the child is awake and under direct supervision.
Testing the skin for tolerance to the 3M™ Micropore™ Tape is worthwhile. Rolls of the Micropore Tape are included in the EarWell Kit. The pre-cut “Top” Retainer Tape is also 3M Micropore Tape. If there is no reaction to this tape, it can be used to secure the ear in lieu of using the Cradle, thereby preserving the initial corrections made by the Cradle and averting regression.
